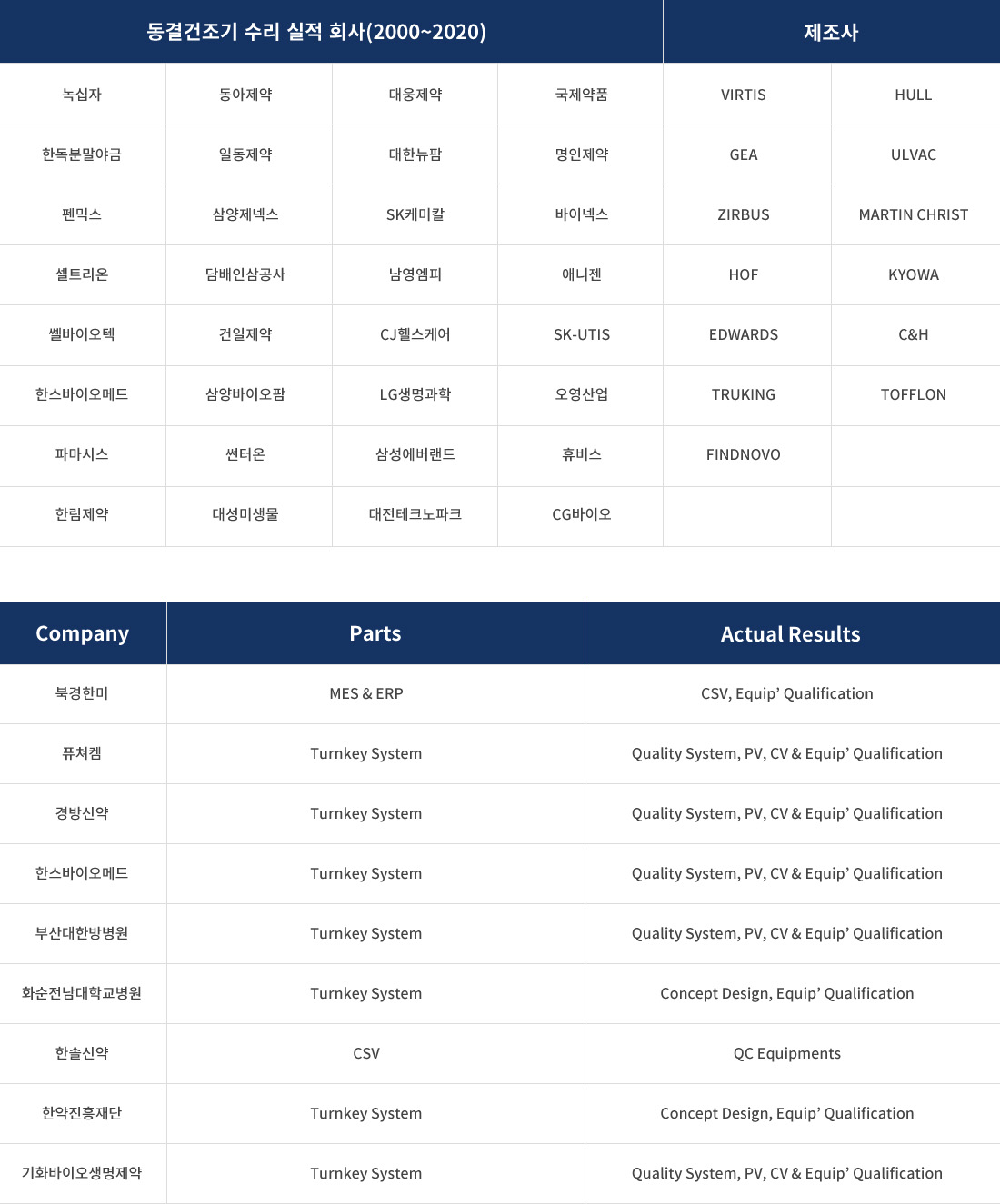
| COMPANY | PARTS | DESCRIPTION | ||
|---|---|---|---|---|
| 에스비피 | EUGMP KGMP |
항암주사제 | Turnkey System | Quality System, PV, CV & Equip’ Qualification |
| Equipments | HVAC System, Autoclave Qualification | |||
| 바이오니아 | EUGMP KGMP |
주사제 | Turnkey System | [Medical Device]Concept Design, Equip’ Qualification, PV, CV |
| Concept Design | [Pharmaceutical] Design for GMP Area | |||
| 인터로조 | JGMP GMP |
점안액 | Turnkey System | Utilities, Laboratory, Manufacture |
| Qualification | Equip’ Qualification | |||
| 한국신약 | JGMP KGMP |
고형제 한방제 |
Turnkey System | Concept Design, Equip’ Qualification |
| 화순전남대학교병원 | KGMP | 방사성의약품 | Turnkey System | Concept Design, Equip’ Qualification, PV, CV |
| 한약진흥재단 | KGMP | 고형제 한방제 |
Turnkey System | Concept Design, Equip’ Qualification |
| 우성양행 | KVGMP | 고형제 항생제 |
Concept Design | Design for GMP Area |
| AK&MN BioFarm | EUGMP JGMP KGMP |
주사제원료 | Turnkey System | Concept Design, PV, CV & Equip’ Qualification |
| Validation System | Quality System, PV, CV & Equip’ Qualification | |||
| 대웅제약 | cGMP KGMP |
주사제 | Turnkey System | Quality System, PV, CV & Equip’ Qualification |
| 선바이오 | EUGMP | 고분자합성 | Turnkey System | Concept Design, Equip’ Qualification |
| 한국신텍스제약 | KGMP | 고형제 한방제 |
Turnkey System | Quality System, PV, CV & Equip’ Qualification |
| L&K BIOMED | KGMP | 세포치료제 | Turnkey System | Quality System, PV, CV & Equip’ Qualification |
| 삼영유니텍 | JGMP KGMP |
방사성의약품 | Turnkey System | Quality System, PV, CV & Equip’ Qualification |
| 샘바이오 | KGMP | 세포치료제 | Turnkey System | Quality System, PV, CV & Equip’ Qualification |
| 삼전순약공업 | KGMP | 원료의약품 | Calibration System | Utilities, Laboratory, Manufacture |
| Turnkey System | HVAC System, PWS, Gas, Manufacture Equip’ Qual’ | |||
| 이글벳 | EUGMP KVGMP |
주사제 고형제 |
Turnkey System | Quality System, PV, CV & Equip’ Qualification |
| Requalification | Autoclave, Dry Heat Sterilizer PQ, Warehouse Mapping Test | |||
| Validation System | Quality System, Equip’ Qualification | |||
| 대정화금 | KGMP | 원료의약품 | Turnkey System | Quality System, PV, CV & Equip’ Qualification |
| Equipments | Reactor System | |||
| 일신케미칼 | KGMP | 원료의약품 | Turnkey System | ALL - PV, CV, & Qualification |
| Equipments | Calibration & Requalification | |||
| Calibration | Utilities, Laboratory, Manufacture | |||
| 켐포트 | cGMP JGMP KGMP |
원료의약품 | Turnkey System | Qualification |
| Requalification | Requal’-HVAC System, Clean Bench, Mapping Test | |||
| KIT(대전/정읍) | FDA GLP |
비임상시험 | Turnkey System | CSV, HVAC, Autoclave 외 |
| Validation System | FDA-흡입독성장비외 CSV, Qual, Calibration | |||
| Qualification/Calib | ALL Equip: CSV, Qualification, Calib | |||
| CSV | Temperature Monitoring System | |||
| 광진테크 | cGMP KGMP |
원료의약품 | Turnkey System | ALL - PV & Equipments Qualification |
| S.Biomedics | KGMP | 세포치료제 | Turnkey System | Re-Validation(BSC, Autoclave, Dry Oven 외) |
| 동덕제약 | KGMP | 주사제 | Turnkey System | ALL- PV, CV, & Qualification |
| SB신일 | EUGMP KVGMP |
주사제 고형제 |
PM | 신축제조소건축업무엔지니어링관리 |
| 휴림바이오셀 | KGMP | 세포치료제 주사제 |
Requalification | Calibration, Equip’ Qualification |
| 종근당 | EUGMP KGMP |
주사제 고형제 |
CSV | Utility System, Manufacture System |
| 한국크리에타(태극제약) | KGMP | 주사제 | Qualification | 충전기 Qualification(DQ~OQ) |
| 휴온스 | JGMP KGMP |
주사제 | Utility System | Validation(HVAC, Air, Gas, Room…) |
| 한국얀센 | cGMP KGMP |
완제의약품 | Calibration | Manufacture Equipments |
| 광림파워팩(태극제약) | KGMP | 완제의약품 | Qualification | Packing M/C IQ, OQ |
| 유영제약 | JGMP KGMP |
완제의약품 | Cal’ System | ALL- Utilities, Laboratory, Manufacture |
| 퍼슨 | KGMP | 완제의약품 | Plant Project | Design & PV, CV, CSV(BMS), Qualification |
| 보령바이오파마 | KGMP | 완제의약품 | CSV/Qual/Cal | BMS, Sterilization System, Equip’… |
| Qualification/Calib | Equipments: Autoclave, All Calibration | |||
| 삼천당제약 | EUGMP KGMP |
완제의약품 | Utility System | Validation(HVAC, Equip, Room…) |
| 영풍제약 | KGMP | 완제의약품 | Qualification/Calib | Equipments: Air System, Calibration |
| 파마킹 | KGMP | 완제의약품 | Cal’ System | ALL- Utilities, Laboratory, Manufacture |
| 삼진제약 | KGMP | 완제의약품 | Cal’ System | ALL- Utilities, Laboratory, Manuf’ |
| 대웅바이오 | KGMP | 원료의약품 | Qualification | Utility System, Manufacture System |
| 건일제약 | KGMP | 원료의약품 | Concept Design | Design for Process Control System, URS |
| 덕산약품공업 | KGMP | 원료의약품 | Calibration | Utilities, Manufacture |
| Calibration System | Utilities, Laboratory, Manufacture | |||
| Sk바이오랜드(오송) | KGMP | 원료의약품 | Calibration | Manufacture Equipments |
| Calibration | Dry Oven, Lyophilizer | |||
| 바이오랜드(천안) | KGMP | 원료의약품 | Equipments | Qualification |
| SEC(대웅바이오) | KGMP | 원료의약품 | Equipments | Dry Oven Qualification |
| Qualification/Calib | Equipments: CSV, Qual, Calibration | |||
| CJ제일제당 | KGMP | 원료의약품 | CSV/Qual/Cal | CMS, Sterilization System, Fermentations.. |
| 동화약품 | JGMP KGMP |
원료의약품 | Validation System | API ALL- Equipments Qualification |
| 유케이케미팜 | JGMP KGMP |
원료의약품 | Equipments | Validation for Sterilization System |
| NKBIO | JGMP KGMP |
세포치료제 | Qualification | Recovery Test |
| Equipments | Revalidation | |||
| Qualification/Calib | ALL Equip: CSV, Qualification, Calib | |||
| 파미셀 | KGMP | 세포치료제 | CSV | Flow Cytometry |
| Plant Project | Design & PV, CV, CSV(BMS), Qualification | |||
| 대한적십자사 | KGMP | 혈액제제 | Validation System | Facilities: Cold Storage System |
| 세브란스병원임상시험센터 | GCP | 고형제 | Re-validation | Utilities, Laboratory Equipments |
| 코오롱제약 | JGMP KGMP |
고형제 | Qualification | HVAC System, BMS CSV |
| Stability Test Chamber Qualification, Utility System Calibration |
||||
| CSV, Install | Building Management System, Wireless Monitoring System | |||
| 한솔신약 | KGMP | 고형제 한방제 |
CSV | QC Equipments |
| Calibration | Utilities, Manufacture | |||
| 나노팜 | KGMP | 연고제 | Equipments | Mixer Qualification |
| Validation System | ALL Qualification(HVAC, Water, Equip) | |||
| MIURA(KIT) | FDA GLP |
비임상시험 | Qualification | Autoclave Calibration, IQ, OQ |
| KITOX(진주) | GLP | 비임상시험 | Equipments | Sterilization Qualification |
| 서울정수(경진제약사) | KGMP | 한방제 | Qualification | Purified Water System IQ, OQ |
| HVLS | KGMP | 한방제 | Qualification/Calib | Equipments: Qual, Calibration |
| 한국존슨앤드존슨 | CGMP | 화장품 | Equipments | Autoclave Validation & Calibration |
| Calibration | Muffle Furnace, Melting Point Apparatus, Microwave, Centrifuge, Fridge-Freezer, Incubator | |||
| 오스템임플란트 | FDA | 의료기기 | Qualification/Calib | ALL Qualification(HVAC, Water, Equip) |
| KFDA | KGMP | N/A | LAB System | 각청시험장비 67대 Validation(IQ, OQ) |
| 김포공항물류센터(보령바이오팜) | KGMP | 물류 | Shipping Validation | Facilities: Cold Storage System |
| 보건의료산업센터 | - | - | Qualification/Calib | ALL Qualification(HVAC, Water, Equip) |
| 충북테크노파크 | - | - | 기업지원프로그램 | 중부권제약사CSV지원사업 |
| 충남테크노파크 | - | - | 기업지원프로그램 | 동물용의약품장비적격성평가지원 |